
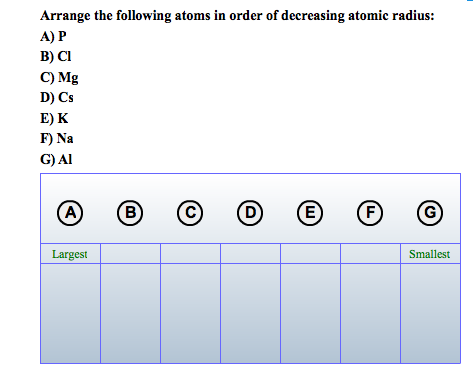
O Positively charged ions are called cations (primary radical), and negatively charged O An ion is a charged species in which an atom or a group of atoms possess a net electric O The number of atoms that combine to form a molecule is called the atomicity of the O A molecule is formed when two or more atoms of the same or different elements combine chemically. The experimental value of 1 gram molecular volume of a gas is 22.4 L at S.T.P. Gram molecular volume: The volume occupied by 1 gram molecule of dry gas at S.T.P is called gram molecular volume. Relative atomic mass or atomic weight is the ratio of the mass of one atom of an element to the mass of an atom of hydrogen taken as unity. The mass of one-mole molecules of any substance equals the gram molecular mass of that substance. U and the gram atomic mass of nitrogen is 14 g. For example, the atomic mass of nitrogen is 14 The mass of one mole of atoms is known as the molar mass ofĪtoms, gram atomic mass, or gram atoms. O The atomic mass of an atom of an element is also known as its relative atomic mass since it is determined relative to the mass of the C-12 isotope. O The mass of an atom is known as the atomic mass. O Symbols are significant as they represent a particular element and one O The element’s symbol is made from one or two letters of the English or the Latin name of the element. The radius of the atom indicates the size of an atom, called the atomic radius. Irrespective of the source of the compound. O A chemical substance always contains the same elements in a fixed proportion by mass, It means that the sum of the masses of the reactants and the products remains the same during a reaction. O Mass can neither be created nor destroyed in a chemical reaction. O An atom can be defined as the smallest particle of matter that can neither be created nor destroyed by chemical means. O Atoms cannot be divided further i.e., atoms are indivisible O Matter is made up of very tiny particles, and these particles are called atoms. 3 Conclusion Atoms And Molecules Class 9th Science: Introduction Atoms According to Dalton’s atomic theory.2 NCERT Solutions for class 9th Science chapter Atoms And Molecules.

1.1.1 According to Dalton’s atomic theory.1 Atoms And Molecules Class 9th Science: Introduction.


 0 kommentar(er)
0 kommentar(er)
